The Search for Water on the Moon
How Lunar Prospector Worked
Dr Jamie Love 1999 ©
1999 ©
 1999 ©
1999 ©
We might still be ignorant about the Moon's water if not for a satellite called Clementine.
Throughout the late 1980s the United States Department of Defense (DOD) was working on a variety of high-tech space projects which most people know by the nickname "Star Wars". Among the various plans was one to use remote-controlled satellites to track, and eventually collide with, targets in space. The military wanted to test their instruments and ideas but they were prevented by certain regulations regarding weapons in space. So they teamed up with NASA to find some common objectives that would have a more "civil" purpose. Space scientists were keen to get a vehicle close to some asteroids in order to collect data that might one day be used to mine them. NASA was happy to help the DOD spend some money on the joint project that was named Clementine (after an old American mining song) to emphasise the potential mining aspect of the project.
|
On the 24th of January 1994 Clementine was launched from
Vandenburg Airforce Base (California, USA). The 140 kilogram satellite
carried an array of instruments including a very good radar system.
After a few weeks in Earth orbit Clementine was sent to the Moon. For two and half months Clementine circled the Moon in a highly inclined orbit, mapping the polar regions in very good detail. That had not been done before by any other satellite and it's impossible to do from Earth. During one particular orbit over the South Pole, Clementine used its radar to measure the reflectance of polarized radio waves. (It's a pretty complicated way to estimate the "texture" of a distant surface.) Data from that single orbit proved to be very interesting! | 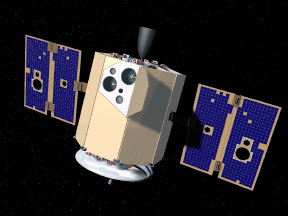 |
On the 3rd of May 1994 Clementine was ordered out of lunar orbit on its mission to rendezvous with an asteroid called Geographos. A computer software bug caused the satellite to lose control and it never achieved its mission object. Clementine never made it to Geographos but the data it collected from the Moon's poles was available for NASA scientists to study.
Some of the scientists interpreted Clementine's radar data as
consistent with the possibility of water ice being in the permanently
shadowed craters near the Moon's south pole in a place called
the Aitken Basin. The Aitken Basin is a giant impact crater and
it's the largest and deepest known crater in the Solar System!
Calculations suggested that the total ice coverage was over 30
square miles, although it was scattered all around the basin.
Other scientists pointed out that the radar data might be interpreted
another way that meant there was no ice there at all. Clementine
had not been designed to look for water on asteroids or the Moon
so Clementine's data was open to different
interpretations and arguments. The only thing that would settle
the argument would be a special mission to observe the lunar poles
with equipment specially designed to look for water.
That's what Lunar Prospector was all about.
|
Lunar Prospector was a 295 kilogram, graphite-epoxy, drum-shaped craft only 1.4 meters in diameter and 1.3 meters high. It had three masts sticking out of it, each 2.4 meters long, to which the sensory instruments are attached. The instruments were placed on the masts to avoid interference caused by signals from the body of the craft. The spacecraft was solar-powered and used rechargeable nickel-hydrogen batteries when it wasn't in the sunlight. Lunar Prospector, or simply "Prospector", was part of NASA's Discovery program to develop missions that are "faster, better, and cheaper". Prospector was created in just 22 months, so it certainly was "fast". Some of NASA's missions have had trouble because they used specially designed and manufactured equipment for each spacecraft. Prospector used "off-the-shelf", flight-proven equipment, and it was designed to get the job done without getting fancy. That makes it "better". Prospector cost a "cheap" $63 million, a small price for almost any spacecraft and a real bargain for starting us on the road to colonizing space! | 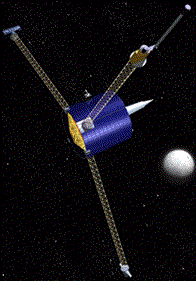 |
On January 11th Lunar Prospector went into a polar orbit 100 kilometers above the Moon. The following year it was ordered into an orbit as low at 10 kilometers to give us even better information. The spacecraft carried five different scientific instruments designed to sense chemical and physical properties of the Moon.
The Magnometer measured both the strength and direction of the magnetic fields around the Moon. These magnetic fields are produced mostly from the Earth and Sun, but when they are subtracted from the data you are left with the magnetic field produced by the Moon. This information will help NASA geologists to map the location of magnetic deposits like iron.
The Electron Reflectometer measured electrons reflected (bounced) from the surface of the Moon. These electrons are reflected off of magnetic materials in a very specific way. (It has to do with some complex geometry.) This instrument complemented the Magnometer as NASA produced its "magnetic map" of the Moon.
The Gamma Ray Spectrometer measured gamma rays (obviously).
Gamma rays are produced when certain radioactive materials undergo
their natural decay, so this instrument was able to map the
locations and concentrations of radioactive materials on the Moon's
surface like uranium and thorium. Perhaps one day these will be
mined to provide nuclear power for missions into deep space where
the use of solar energy would be impractical.
Gamma rays are also produced by nonradioactive elements when they
are struck by powerful cosmic rays so, Prospector's
Gamma Ray Spectrometer could be used to sense nonradioactive elements
too. Cosmic rays are not rays at all (!), they are extremely
high-speed, subatomic particles. Cosmic "rays" are common
in space, falling onto the Moon (and Earth and everything else)
from all directions, so they are constantly striking materials
on the Moon's surface and producing gamma rays that Prospector
can detect.
The interesting thing about a gamma ray, regardless of how it is produced, is that the energy of the
gamma ray tells you what element emitted it. So this device
allowed NASA scientists to map the location of uranium and thorium
(directly by the gamma rays they emit as they decay) and iron,
oxygen, silicon, aluminum, calcium, magnesium, and titanium (indirectly
by the gamma rays they produce when hit by cosmic rays).
The Alpha Particle Spectrometer measured another product of radioactive decay. Alpha particles are the nuclei of a helium atom (two protons and two neutrons). Although not as versatile as the Gamma Ray Spectrometer, this instrument worked in a similar way and gave us information about the small amounts of radioactive gases released from the Moon. Gases released from a planet are an important geological phenomenon, called outgassing. The Alpha Particle Spectrometer provided more clues about the Moon's composition and evolution.
The Neutron Spectrometer measured neutrons and was the most important instrument on Lunar Prospector because it's the gizmo that proves there is water on the Moon!
How? Neutrons are just neutral atomic particles. How can you sense neutrons?
Good question. You do it indirectly. Let me tell you how Prospector's Neutron Spectrometers detects neutrons.
First you should know that it isn't really one instrument but two almost identical instruments. They are both tiny canisters containing a special gas called helium-3 (or 3He to use the proper symbols). 3He is made of two protons and one neutron. The two protons give the atom its "elemental characteristics". That is, these atoms are helium atoms because they contain two protons in their nucleus. The neutron doesn't really influence any chemical properties so it's easy to think of it as just extra mass. (Which it is!) However, it gives 3He special nuclear properties.
|
When the 3He is hit by a neutron it exchanges one of its protons for
the neutron.
The result of this nuclear reaction is that the 3He is transformed into a new element (hydrogen-3 or 3H, an isotope of hydrogen called "tritium"). In the process a proton and lots of energy are released. The burst of energy is detected and counted. | 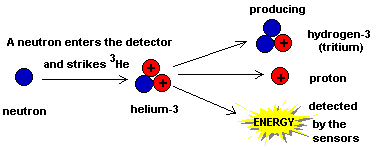 |
OK, that's how Prospector detected neutrons, but what's the point of two different containers?
|
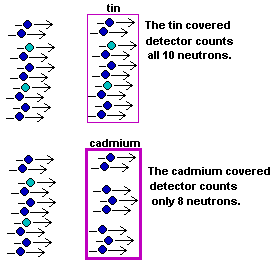
One container is coated with tin that allows all neutrons, even
very slow moving ones, to enter. The other container is coated
in cadmium, a metal that screens out the slow moving neutrons,
so only fast (high energy) neutrons can get through.
This means you can use the two containers, side by side, not only to detect a group of incoming neutrons, but also to tell if those neutrons are moving fast or slow. Together the two containers allow you to divide the population of neutrons into high energy and low energy. That's what a "spectrophotometer" is all about! | 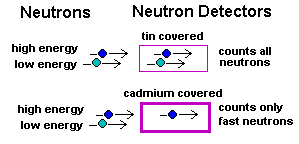 |
Separately you have two neutron detectors, one more sensitive than the other, but both are merely detectors because all they do is detect and count neutrons. Together you have a spectrophotometer because by using the two together you can determine the amount of both levels of energy in a shower of neutrons.
|
Imagine a group of neutrons hits Prospector's Neutron Spectrophotometer
(both detectors).
Suppose the tin detector, the more sensitive detector, counts 10 neutrons. (Actually the detector counts the burst of energy produced by the nuclear reaction described above, but you know what I mean.) Also, suppose the cadmium container
counts only 8 neutrons.
By using the data from both containers you can say that
the population of neutrons that hit Prospector's Neutron Spectrophotometer
is composed of 80% high-energy and 20% low-energy neutrons.
|  |
So, what does this have to do with water?
Everything.
To put it all together you need to learn where the neutrons come from and how they behave when they hit something.
You'll recall that cosmic rays are all over the place and they constantly bombard the surface of the Moon producing gamma rays which Prospector's Gamma Ray Detector can use to map the location and concentration of elements that don't normally produce gamma rays (like iron, silicon, etc.). Well, that was only part of the story. When cosmic rays strike an atom they not only release gamma rays but also dislodge neutrons that go flying out of the atom at a very fast speed. We call these very fast neutrons "hot" neutrons. Most of them fly off into space but some of them will collide with materials in the Moon's surface and are slowed by the collisions. Because the collisions slow them down we say the neutrons have "cooled", or their speed has been "moderated". How much they are moderated (cooled) depends upon what they hit. This has to do with momentum and is best illustrated with marbles!
|
Think about how you play a game of marbles. Recall how a marble behaves when it hits a stationary marble. Both, being similar in mass, will fly off in opposite directions. The stationary marble will move away at half the speed of the striker, which moves away at half its original speed. On the other hand, if you rolled the marble against a bowling ball it would not budge, and the marble would fly off at pretty much the same speed it had to begin with.
Imagine this "marble game" at the subatomic level with
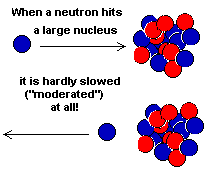
a neutron playing the part of the incoming marble. If a neutron
hits an atom with a large nucleus the neutron will bounce off
with only a slight loss in speed. Its speed will have been moderated
only slightly. On the other hand, if the neutron hits a small
nucleus it will be slowed down more because it will transfer more
of its energy (momentum) to its target.
|  |
OK, last time! What does this have to do with water??!!!
Everything.
Water is made of one oxygen atom and two hydrogen atoms (H2O). Hydrogen is the simplest atom in the universe because hydrogen is made of only a single proton. (OK, it can sometimes have a neutron or two but that's rare.) Protons and neutrons are identical in mass (almost). When a neutron hits a hydrogen nucleus (a proton) it bounces off at half its original speed. If it bounces off another hydrogen it loses half its speed again. The more hydrogen atoms in the neighborhood the more the neutron collides with them, and the slower or "cooler" it gets. Hydrogen in the water is a perfect neutron moderator!
Now is a good time to put all these thoughts together.
 |
Neutrons bounced off any element other than hydrogen are moderated
only slightly. Scientists call these medium energy neutrons "epithermal"
neutrons and both detectors can sense them.
Neutrons bounce off a hydrogen nucleus at half their original speed. The more water around them, the more the neutrons' speed is moderated. Scientists call these slow neutrons "thermal". | 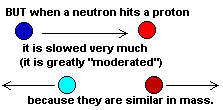 |
These slow, low energy, thermal neutrons cannot pass through the walls of the cadmium detector but they can make it though the walls of the tin detector.
When Prospector passes over a crater containing ice it will detect
an increase in the number of thermal neutrons and a decrease
in the number of epithermal neutrons, because the hydrogen
in water causes the neutrons to become more moderated.
(Read that sentence
again and make sure you understand it.)
Wait a minute. Water isn't the only thing that contains hydrogen.
That's right. Here on Earth we find hydrogen in lots of different
things, especially living things, but hydrogen doesn't combine
well with materials like rocks. On the surface of the Moon there
is very little that hydrogen can bind to except oxygen. So water
is the most likely source of the hydrogen signal.
Note: the "solar wind" can also "stick" hydrogen
to the surface of the Moon by different means, but this hydrogen
doesn't amount to much.
Prospector's Neutron Spectrometer was a very sensitive device and it was able to detect water hidden slightly below the surface (about half a meter down). That's because the cosmic rays that produce the neutrons can penetrate pretty deep and the neutrons they produce can bounce around a lot before making their way to the surface. NASA scientists estimate that Prospector's Neutron Spectrometer could sense a cup of water distributed in a cubic meter of lunar "soil".
|
The vertical axis is the number of medium energy (epithermal) neutrons detected.
The horizontal axis shows the location (latitude) at which Prospector collected those neutrons. Notice that more epithermal neutrons were detected along the Moon's equator ("EQ" or Latitude 180) than at the North Pole (latitude 90 degrees) or South Pole (latitude 270 degrees). Recall that cosmic rays hit the Moon from all directions so neutrons must be produced at the same rate all over the Moon. Prospector's detector counted fewer medium energy neutrons at the poles because something there is slowing down the neutrons, moderating them. Water hydrogen is the most likely explanation for this moderation of neutrons. |  |
Notice that there are dips at both poles. Clementine's data suggested that there was water only at the South Pole (Aitken Basin) but Prospector proved there is water at both poles. Also, notice that the dip in the number of neutrons is about the same in both poles (reaching a low of about 585 neutrons) but the dip is more pronounced at the North Pole than at the South Pole. NASA scientists estimate that there is about 50% more water at the North Pole than at the South Pole.
Note: NASA has been able to use Lunar Prospector's Gamma Ray Spectrometer to add to the epithermal neutron data because it had a "shield-detector" (which I won't go into) allowing it to detect epithermal neutrons. So Prospector had TWO ways to measure epithermal neutrons.
Another hidden feature in the data is that the amount of fast (hot) neutrons detected indicates the "mixing ratio" of the ice in the "soil". In other words, it lets you know if there are solid chunks or tiny crystals of ice. Data from Prospector (not shown here) indicate that the water is in a very low mixing ratio, between 0.3% to 1.0%. That means most of the water is not chunks of ice but tiny crystals dispersed over a large area. To put it another way, the ice in the permanent shade is not ice puddles but a "permafrost"! That might make it more difficult to collect but at least we now know what to expect.
By carefully measuring the amount of "missing" epithermal neutrons, using much more data then shown in the above graph, NASA estimated the amount of water on the Moon at 10 to 300 million cubic meters (or 2.6 to 26 billion gallons, if you're old fashioned). Prospector has certainly shown there's a lot of water on the Moon and provided more useful data for our colonization of the Moon.





If you have a comment or question about lunar water feel free to send a Letter to the Editor. ![]()
I can't promise to answer all your questions or address all your comments, but I'll post a few particularly good ones here.
Return to the Science Explained Homepage.