The Martian Fossils
Dr Jamie Love 15 January 1998 ©
15 January 1998 ©
 15 January 1998 ©
15 January 1998 ©
| In the summer of 1996 scientists announced they had found evidence of life in a meteorite from the "red planet". One bit of evidence was a tiny shape they called "the worm". That's the kind of thing most people think of when they think "fossil". But most paleontologists (fossil scientists) are not impressed by "the worm". ALL the remaining evidence is chemistry! | 
|
Much of that chemistry has to do with isotopes. The careful measurements
of isotopes are the best evidence that the meteorite came from
Mars. Isotopes of the same element have the same chemical behaviour, but slightly different mass.
For example, if you replaced an atom in a molecule with another
atom of the same element but of a different isotope, the new
molecule would have all the chemical properties of the original
molecule. However, it would have a different MASS because you
switched isotopes.
It's important to understand that the change in mass of a molecule caused
by switching isotopes is often very small in comparison with the
mass of the entire molecule or a sample (a "bunch" of molecules).
Think about a fairly small molecule, water. The most common molecules
of water (H2O) have a molecular mass of 18 Daltons (16 from oxygen
and one each from hydrogen). If you were to substitute a deuterium
atom (an isotope of hydrogen with a mass of two Daltons) for one of the normal hydrogens (with a mass of only one Dalton), you would increase the
total mass of the molecule by only a single Dalton. The (new)
heavier water would have a mass of 19 Daltons. Its mass would have
increased by only 5%.
Now imagine you put that one odd molecule of heavy water into
a raindrop containing billions of the normal water molecules.
The change in mass for the whole collection of water molecules,
the whole raindrop, would be incredibly low.
Yet, there are machines which can separate those molecules or atoms entirely by that difference in mass of just one Dalton, and there are detectors that can count them. They are called mass spectrophotometers, but most
chemists call them simply "mass specs". Mass specs can detect the tiniest differences
in the mass of atoms and molecules.
(A speck of a difference in mass!)
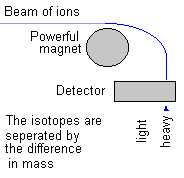
|
This diagram shows how a mass spec works. Ions (atoms or molecules without their normal number of electrons) are shot past a strong magnet and their paths are bent by its magnetic field. The less massive the ion the more its course is bent. The more massive the ions the less they are deflected from their path.
This idea is really
simple to understand. A car of small mass is more easily deflected
from its path than a heavier car. Right? It's called inertia and
it applies equally well to cars or ions. (Because cars and ions are matter and all matter has mass.)
|
Hey, what's this got to do with proving this meteorite is from Mars?
Well, mass specs were sent to Mars many years ago to measure the Martian elements and isotopes. Space chemists soon learned that some of the isotope ratios were very different from ratios found anywhere on Earth! (And they looked everywhere!)
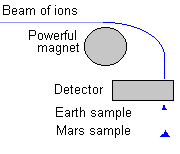
|
For example, the ratio of deuterium to hydrogen (D/H ratio) on Mars is five times that on Earth. Martian water is heavier, more massive, than Earth water! Also, there is more of the heavier isotope of nitrogen (N) in the Martian atmosphere; 60% "enriched" over the 15N/14N ratio found on Earth. There are other elements which have other isotope ratios not found on Earth. This list of isotope differences is called an "isotope signature". |
Some chemists decided to crack open some meteorites and
measure the isotope ratios of the gases released.
These rocks were obviously
meteorites (from "outer space") because they had been
found on top of glaciers in Antarctica. Rocks can only get on
top of a glacier by being dropped from the sky. They found one rock
that had the same isotope ratios as Mars! Then they found another.
Now they have identified about a dozen meteorites with Martian
signatures.
One of these rare meteorites, called ALH84001, was examined further.
By the way, this meteorite was "named" ALH84001 by the scientists
who found it. It was found in the Antarctica Lowland Hills (ALH) in 1984 (84) and was the first one collected that year (001).
Inside ALH84001 space geologists found "the worm" and a few other less
interesting things they called "fossils". The rock also
had a variety of chemicals inside which might (or might not) have been
made by previously living things!
Meteorite ALH84001 is definitely from Mars. The evidence is in the isotopes. It has a Martian signature. But does meteorite ALH84001 contain fossils? Chemicals are the clue.
The research paper which described "the Martian fossils" starts with this sentence, "Fresh fracture surfaces of the martian meteorite ALH84001 contain abundant polycyclic aromatic hydrocarbons (PAHs)." But exactly what does this sentence mean and what are they talking about?
"Fresh fracture surfaces" refers to the inside of the meteorite. It means they broke it open (made a fresh fracture) exposing a new surface which has not seen the light of day since the rock was made on the surface of Mars billions of years ago. Inside this rock, on these "fresh fracture surfaces", chemists discovered lots of polycyclic aromatic hydrocarbons, or PAHs (because even chemists get tired of long-winded words!). These PAHs are the best evidence of previous life on Mars and a critical part of the story.
So what are they?
|
PAHs are large, organic molecules. We often draw organic molecules without the hydrogens
in order to make them look better.
The "H" in PAH stands for hydrocarbon, so you should know that they are talking about molecules containing nothing but hydrogen and carbon. Hydrocarbons are a fairly simple area of organic chemistry. Most hydrocarbons are strings of carbon with hydrogens dangling all along it. But these particular hydrocarbons are "polycyclic" (the "P" in PAH). Polycyclic hydrocarbons are hydrocarbons with lots of rings ("poly" means "many" and "cyclic" means "rings"). The "A" stands for "aromatic" and refers to (hydro)carbon rings with many double bonds. They have a distinct aroma. |
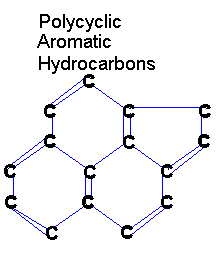
|
So PAHs are polycyclic aromatic hydrocarbons. Or simply, rings of hydrocarbon with lots of double bonds. PAHs are often all that remains of living things after billions of years of decomposition.
The NASA chemists identified the PAHs using a "microprobe two-step laser mass spectrometer". You are now familiar with mass specs as described earlier, but a "microprobe two-step laser mass spectrometer" is more advanced and more sensitive. (I wish I had one!) This "gizmo" uses lasers to push on molecules. From the amount of effort it takes to push the molecule they can figure out its mass. Unlike the mass spec discussed earlier, this "microprobe two-step laser mass spectrometer" measures the mass of large molecules, not just atoms! And it tells you how much is there. Another nice feature of this type of mass spec is that it allows you to scan across the surface at very high magnification. It's a "microprobe" and it's particularly good at finding PAHs.
|
By scanning the fresh fracture surfaces
they collected mass specs on 1,280 different parts of the meteorite's
interior. Then they added up all those bits of data to produce
this average mass spec. The "signal intensity" is a
measure of how common each molecule is. Their diagram shows each
molecule's abundance by the height of the peaks on the graph.
The position of the peaks along the horizontal corresponds to
each molecule (separated by mass).
They used this mass spec to identify a group of organic molecules.
Some of the more important ones include
|
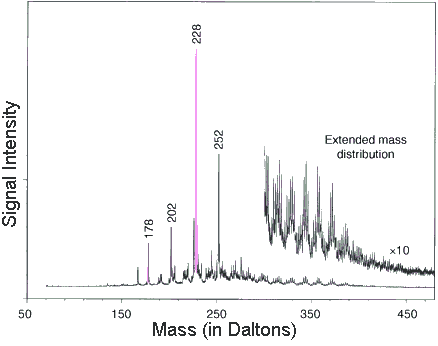
|
When they magnified (by ten times) the right side of the graph (from 300 to 450 Daltons) they found evidence of even larger PAHs.
However, the chemists who identified these molecules in the Martian meteorite were concerned that the PAHs they found may have been introduced to the rock AFTER it fell to Earth. PAHs are very common on our planet because they are very common products of life on Earth (and maybe elsewhere too).
If the PAHs were from Earth life, you would expect them to be more common near the surface of the rock, not deep inside. This is a matter of scientific deduction. If the meteorite had come to Earth without PAHs, then any PAHs added by Earth-life would have been more concentrated at the surface. That's because the Earth-life would first cover the outside of the rock before tunneling in. (Think about it. Think about how moss and lichens cover rocks.)
|
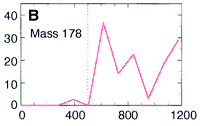
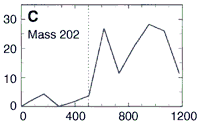
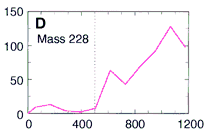
So they used their microprobe two-step laser mass spectrometer
to look at the occurrence of these PAHs at various depths
within the meteorite down to 1200 microns deep (about 1/16 of an inch).
Here's their data, showing how three PAHs INCREASE in concentration as they went DEEPER into the meteorite. The numbers along the horizontal are the depth in microns. (Microns are a millionth of a meter.) This increase in PAHs as they probed deeper into the meteorite is good evidence that the PAHs were from Mars and not from Earth. The PAHs that had been on the surface of the meteorite were probably burned off as the rock passed through space and the Earth's atmosphere. Only the PAHs buried safely inside the rock made it to our world. The low number of PAHs on the surface proves the PAHs didn't leak in from the outside. Because the distribution of these PAHs increases as they scanned deeper, they must have been put there before the rock came to our planet. NASA's chemists (and colleagues) also showed other evidence to support their argument that these molecules are from Mars. Most chemists are convinced these PAHs are from Mars. But are they the chemical fossils of long dead Martians? Not all chemists agree. Space chemists have known for many years about PAHs from outer space. They're common! PAHs have been found in "space dust"; tiny interplanetary and interstellar particles which drift through space for billions of years and for many billions of kilometers. PAHs have also been found in meteorites known to come from the asteroid belt. But few chemists have ever claimed those PAHs to be signs of life. Many space chemists believe them to be made from other, non-life, chemistry. "So what?!", they ask, "Not all PAHs come from living things.", and they're right. Even the NASA chemists agree that there may be other non-life explanations for the PAHs. However, they point out that the PAHs are not just mixed randomly in the meteorite's interior, "The PAHs were found in highest concentration in regions rich in carbonates." |
|
Carbonates?
Yes! Some chemists believe these "regions rich in carbonates" are ANOTHER chemical Martian fossil. That would mean TWO types of evidence for fossils lying side by side - very good support for them being true Martian fossils. But other chemists disagree. They say that simple geological processes on Mars made the carbonates; it has nothing to do with life. They go on to present evidence that the carbonates were formed under conditions which were impossible for life (as we know it)!

| Most of the tiny Martian "fossils" are surrounded by carbonates. Carbonates are anions (negative ions because they have "too many" electrons) made of carbon and oxygen and have the chemical formula CO3-2. The researchers (McKay and his friends) proved that the carbon and oxygen isotopes show a Martian signature; their isotopes are in the ratios expected of Martian carbonates, not Earth carbonates. So these carbonates are definitely from Mars. |
In order to get a good look at these carbonate globules, the researchers used a wonderful technique called "backscatter electron (BSE) imaging". Electrons are fired at the object and those that bounce back (backscattered) at used to create an image. BSE (not to be confused with the prion disease with the same initials) can be used to make clear, high magnification images. The best part about BSE is that the energy of the electrons which bounce back depends upon the type of atom from which they are bounced. So BSE not only lets you see the tiny object but also tells you what elements it's made of.
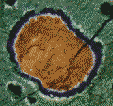
| Using BSE the researchers were able to "see" what cation (positive ions because they have "too few" electrons) is associated with the carbonates and thus give a chemical structure to these globules. They found that the globules have calcium (Ca) rich cores, but also contain very high amounts of manganese (Mn). These are surrounded by layers of iron (Fe) and magnesium (Mg) rich bands. This pattern of alternate layering (along with a few other patterns involving the sulfur chemistry) is very similar to the pattern of carbonates made by bacteria in labs and ponds on Earth. |
Living things tend to alter their immediate environment because life is a complex series of reactions. The respiration of bacteria changes the environment creating a sphere of "reduced" chemicals around the bacteria. These conditions cause carbonate molecules to form in concentric rings; that is, rings inside of rings. The interior carbonate globules are dominated by manganese and calcium carbonates; these are then followed by rings of iron carbonate which are followed by iron sulfides. McKay and his partners explain that the pattern of concentric chemical shells found in the Martian meteorite, "... is a sequence that is common in terrestrial [Earth] settings, because Mn is reduced first by biogenic [living] action, followed by ferris iron and sulfate."
Can these shells of chemicals be made by things that are not alive?
| That's a good question. The answer is, yes! Hot water passing through rocks here on Earth can create these shells of chemicals. Perhaps hot water (warmed by now extinct Martian volcanoes) circulated through the Martian crust and deposited this sequence of chemicals. Martian geology, not Martian biology, might explain these interesting shells of minerals. | 
|
Very high temperatures are required to create these shells by geochemistry alone. Those temperatures would be far too high for life itself (as we know it) to exist. On the other hand, Martian microbes might have had the potential to make those shells (by their metabolism) at comfortable temperatures. Either life (at low temperatures) or geology (at high temperatures) made those mineral shells and no one has found a clear way to tell them apart. The details based upon the thermodynamics of chemical reactions are too advanced to detail here.
The carbonate chemistry of the Martian meteorites is not the final evidence.
Using more BSE images McKay and friends found magnetic iron compounds associated with the "fossils". The way these compounds are arranged around the "microfossils" (their "spatial distribution") is very similar to the way these chemicals are found around "magnetofossils".
"Magnetofossils?"
Yes. Some of Earth's bacteria are magnetic! They use their metabolism to precipitate crystals of iron oxide, called "magnetite". These crystals act as magnets and allow the bacteria to know which way is down. If you thought a magnet only points north-south, you haven't really considered the shape of the Earth. The closer your compass (or magnetic bacteria) are to a pole, the more the "needle" bends down towards the ground. The magnet follows the magnetic field as it dips into the Earth. Bacteria which live in "anaerobic" (low oxygen) conditions swim to the bottom of a pond where the conditions are better for their growth. When the bacteria die they leave behind their magnet as a fossil; a magnetofossil.
|
McKay's publication explains that the, "Magnetite particles
in ALH84001 are similar (chemically, structurally, and morphologically)
to terrestrial magnetite particles known as magnetofossils ..."
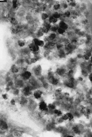
To the right is an image of one of the Martian "magnetofossils". There's not much to them. The tiny black crystals are the magnetite. We know that Mars has a very weak magnetic field today; but long ago it might have had a more powerful one. Perhaps the Martian bacteria used it to find their way home. Or, perhaps the magnetite is just another interesting mineral formed by natural geological chemistry. It happens all the time on Earth. You don't need bacteria to make magnets. |
|
In their important research paper, McKay and his friends pile on the chemistry to convince us that these tiny bits of Martian material are the remains of long dead Martian life-forms. The isotopic data proves these structures are from Mars. And the detailed microchemistry (PAHs, carbonates and magnetic structures) looks remarkably like the microchemistry we find in Earth microfossils. The researchers end their paper with this careful conclusion,

| "None of these observations is in itself conclusive for the existence of past life. Although there are alternative explanations for each of these phenomena taken individually, when they are considered collectively, particularly in view of their spatial association, we conclude that they are evidence for primitive life on Mars." |
The idea that Mars may once have been alive is an exciting thought. It would be the proof that so many scientists have been searching for. Most scientists agree that life probably evolved on worlds other than Earth. Indeed, there are scientists, called exobiologists, who search for extraterrestrial life. If they find what they are looking for our view of the universe would be changed. It would be extraordinary! But, as the Earth's most famous exobiologist, Carl Sagan, once said, "Extraordinary claims require extraordinary evidence."
I (and most other scientists) think the chemistry of ALH84001 is interesting but it isn't proof of life on Mars. (Sorry McKay.)



If you have a comment or question about the "Martian fossils" feel free to send a
Letter to the Editor. ![]()
I can't promise to answer all your questions or address all your comments, but I'll post a few particularly good ones here.