|
Lesson Thirty Six
by Dr Jamie Love |
|
 and
and  licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.

|
Lesson Thirty Six
by Dr Jamie Love |

|
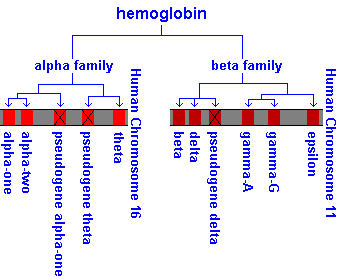
Disorders of human hemoglobins - hemoglobinopathies - are the most common genetic disease group in the world (5% of people are carriers) and cause substantial morbidity (about 300,000 born each year).
Hemoglobin is a tetrameric molecule composed of four subunits
(globins) and a prosthetic heme group which binds oxygen. Each tetramer is composed of two alpha( )-like globin chains (encoded on chromosome 16) and
two beta (
)-like globin chains (encoded on chromosome 16) and
two beta ( )-like globin chains (encoded on chromosome 11), that form a
globular tetramer of 64.5 kD.
)-like globin chains (encoded on chromosome 11), that form a
globular tetramer of 64.5 kD.
Note that hemoglobin is NOT an enzyme so heterozygotes often display, perhaps only a hint of, something "wrong". Hemoglobin is not a structural protein like collagen but, like collagen, it is the product of two genes, both of which makes products that form heteromers. In the heterozygote, combinations of healthy and unhealthy products, in combination with the other protein that makes the heteromer, produce a variety of different tetramers based upon the stoichiometric effects.
There are several copies of human hemoglobins at these two positions and they are developmentally regulated.
Two important points, one about each locus, complicate matters.
1) There are TWO  globins (
globins ( 1 and
1 and  2) and they are equally expressed "forever". Most people, including me, simply think of them as contributing "
2) and they are equally expressed "forever". Most people, including me, simply think of them as contributing " globin" and think about these two closely linked genes separately only when there is something like a mutation to consider. (There are also two copies of gamma globin but this is not as important and rarely relevant to pathologies.)
globin" and think about these two closely linked genes separately only when there is something like a mutation to consider. (There are also two copies of gamma globin but this is not as important and rarely relevant to pathologies.)
2) Almost immediately during embryo development a "competition" begins at the  -like locus between the "fetal"
-like locus between the "fetal" 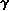 globin (gamma globin) and the "adult"
globin (gamma globin) and the "adult"  globin. Initially
globin. Initially 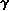 globin wins in that it is the gene most transcribed.
globin wins in that it is the gene most transcribed.  globin can be detected in early gestation but its synthesis becomes
significant only near the time of birth. By 3 months of age (after birth), almost all the transcription from the
globin can be detected in early gestation but its synthesis becomes
significant only near the time of birth. By 3 months of age (after birth), almost all the transcription from the  -like locus is
-like locus is  globin. This switch probably results from a switch in the organ of hematopoiesis as it goes from liver to bone marrow. More on that shortly.
globin. This switch probably results from a switch in the organ of hematopoiesis as it goes from liver to bone marrow. More on that shortly.
These globins have 7 or 8 helical regions, depending upon
the specific globin, and the confirmation of these helixes is conserved - although there is divergence of the primary sequence. Indeed, only two particular amino acids have been conserved in all
the globins (found throughout nature, including in the root nodules
of legumes) - the histidine that is covalently bound to the iron of the heme and a phenylalanine that forces the heme's porphyrin into its position in the protein.
Any mutation that alters globin confirmation or these specific two amino acids, is likely to cause a hemoglobinopathy.
Gene switching is the change in expression of genes during development and occurs in many systems but the gene switching in hemoglobin is a fine example (and useful to understand because the basic concepts can be more broadly applied).
Genes in each of the two globin clusters are expressed sequentially but there is equimolar
production of the two  -like and two
-like and two  -like globin chains.
-like globin chains.
The first (and transitory) expression of globin is the
 -like
-like 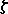 globin (theta globin) and
globin (theta globin) and  -like
-like  globin (epsilon globin) which together
are synthesized in the yolk sac from the third to eighth
week of gestation.
globin (epsilon globin) which together
are synthesized in the yolk sac from the third to eighth
week of gestation.
At about the fifth week, the fetal liver becomes the major site of hematopoiesis. Expression in the liver is different from that in the yolk sac. In the liver,  globin is transcribed from the
globin is transcribed from the  -like cluster (not
-like cluster (not 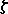 ) and
) and 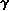 is transcribed from the
is transcribed from the  -like cluster (not
-like cluster (not  ).
).
Synthesis of 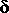 globin (delta globin) begins in late fetal life, probably in the
bone marrow only, and continues after birth, but it never accounts for more than 2% of the transcription at the
globin (delta globin) begins in late fetal life, probably in the
bone marrow only, and continues after birth, but it never accounts for more than 2% of the transcription at the  -like locus.
-like locus.
The normal expression of genes in the  -like cluster requires a
domain called the "locus control region" (LCR), positioned about 10 kb upstream of the
-like cluster requires a
domain called the "locus control region" (LCR), positioned about 10 kb upstream of the  globin gene. Patients with deletions of the LCR fail to express the genes of the
globin gene. Patients with deletions of the LCR fail to express the genes of the  -like cluster and die as embryos.
-like cluster and die as embryos.
Due to the complex switching of genes and organs of hematopoiesis during development it is often difficult to keep track of the definitions and sequence of events. Use the table and diagram below to help you define the different types of hemoglobin found throughout development. (Remember, the timing is roughly correct BUT switching doesn't happen instantaneously - it smoothly transits.)
|
 |
Keep in mind that there is a transition occurring here. Hb F is the predominate hemoglobin throughout fetal life and makes up 70% of total hemoglobin at birth, but in the adult Hb F represents less than 1% of the total hemoglobin. Hb A2 never accounts for more than 2% of adult hemoglobin.
NOTE(S):
1) Mutations in the one  globin gene are more likely to produce a pathology than mutations in one of the two
globin gene are more likely to produce a pathology than mutations in one of the two  globin genes. (The
globin genes. (The  globin genes have built in redundancy because there are two of them.) Think about it. A
globin genes have built in redundancy because there are two of them.) Think about it. A  globin mutation will affect half of the chains produced (the other half are from the other chromosome which is unaffected in a heterozygote). But a single
globin mutation will affect half of the chains produced (the other half are from the other chromosome which is unaffected in a heterozygote). But a single  globin mutant will affect only a quarter of the globin chains (because there are two copies -
globin mutant will affect only a quarter of the globin chains (because there are two copies -  1 globin and
1 globin and  2 globin).
2 globin).
2)  globin mutations do not cause any prenatal problems because
globin mutations do not cause any prenatal problems because
 globin is the major
globin is the major  -like globin before birth. Hb F makes
up 70% of the hemoglobin at birth.
-like globin before birth. Hb F makes
up 70% of the hemoglobin at birth.
3)  globin mutations affecting BOTH copies of
globin mutations affecting BOTH copies of  globin (say a large deletion that removes them both) can harm both fetal and postnatal life (because
globin (say a large deletion that removes them both) can harm both fetal and postnatal life (because  chains are the only
chains are the only  -like component of all the hemoglobins 6 weeks after conception).
-like component of all the hemoglobins 6 weeks after conception).
Hereditary hemoglobins disorders are characterized into three groups.
1) Structural variants alter the globin protein. This is a structural mutation.
2) Thalassemias have an imbalance in the relative amounts
of  and
and  subunits. This is a mutation causing a decrease in the synthesis of a globin.
subunits. This is a mutation causing a decrease in the synthesis of a globin.
3) Hereditary persistence of fetal hemoglobin (HPFH) is due to impairment of the perinatal switch from  to
to  globin. It is not of clinical significant (but interesting and worth knowing about).
globin. It is not of clinical significant (but interesting and worth knowing about).
There are more than 400 abnormal hemoglobins (mostly resulting from point mutations) and about half are clinically relevant. These structural variants can produce two clinical phenotypes.
1) Hemolytic anemia is usually caused when the molecules assume (usually polymeric) ridged structures, but a variety of other anemias are due to unstable molecules.
2) Altered oxygen transport is due to changes in the oxygen affinity or due to the formation of methemoglobin (which is incapable of reversible oxygenation).
Sickle cell hemoglobin (Hb S) is caused by a point mutation (a single A->T) causing an amino acid substitution (Glu->Val) in the 6th position of  globin's 146 amino acid chain (
globin's 146 amino acid chain ( 6 Glu->Val).
6 Glu->Val).
Note: the endonuclease MstII recognizes the normal sequence and cleaves
it, but does not touch this mutant gene. This is very useful
for diagnostics (including prenatal from CVS) because you can
use Southerns (or MstII digested PCR fragments) of the
 globin fragment to distinguish homozygotes, carriers and normals.
globin fragment to distinguish homozygotes, carriers and normals.
Homozygotes for Hb S have sickle cell disease, a severe
hemolytic condition. Low oxygen conditions cause this hemoglobin
to aggregate into rod-shaped polymers which distort the shape
of the RBC to a sickle shape. These cells cannot squeeze
though capillaries in single file and therefore block blood flow
causing local hypoxia. This causes damage to a variety of tissues
and organs. Recurrent infarctions in the spleen ultimately cause the organ to regress
during childhood and this causes immune dysfunction. (Infection
is the major cause of death.)
Treatment is only supportive.
Sickle cell trait occurs in the heterozygotes, who are clinically normal, but their cells will sickle when subjected to low oxygen and there is a risk of splenic infarction when at high altitudes (mountain climbing or in unpressurised planes). Recall that these heterozygotes are also resistant to Plasmodium vivax (a selective advantage).
Protein electrophoresis can easily distinguish between HB A (the normal adult hemoglobin) and HB S and a heterozygote shows a mixture of the types of hemoglobin made from subunits of A and S.
 2 2 A2 A2 |  / / and and  / / | ||
 2 2 A2 and A2 and  2 2 A A  S and S and  2 2 S2 S2 |  / / and and  / / S S | ||
 2 2 S2 S2 |  / / and and  S/ S/ S S |
Hemoglobin C (Hb C) is (coincidentally) also due to a mutation
at the 6th position of  globin, but this time replacing the glutamate with lysine
(
globin, but this time replacing the glutamate with lysine
( 6 Glu->Lys). This hemoglobin is less soluble than Hb A so it crystallizes in RBCs reducing their deformability in capillaries. This problem causes only a minor hemolytic disorder.
6 Glu->Lys). This hemoglobin is less soluble than Hb A so it crystallizes in RBCs reducing their deformability in capillaries. This problem causes only a minor hemolytic disorder.
Compound heterozygotes for the  C and
C and  S mutations have Hb SC disease, a mild hemolytic disorder which may have no clinical consequences. However, a serious vascular occlusion often occurs producing a retinopathy.
S mutations have Hb SC disease, a mild hemolytic disorder which may have no clinical consequences. However, a serious vascular occlusion often occurs producing a retinopathy.
An example of an unstable hemoglobin is Hb Hammersmith which is usually due to a point mutation which causes denaturation of the globin. The denatured globin chains are insoluble so they precipitate to form inclusions called "Heinz bodies". These Heinz bodies damage the RBC's membrane causing hemolysis.
Note: the most common point mutation of Hb Hammersmith substitutes a serine for the invariant phenylalanine at position 42 of the  globin (
globin ( 42 Phe->Ser). So, in addition to its instability, people with Hb Hammersmith often have hemoglobin with a low oxygen affinity, causing cyanosis.
42 Phe->Ser). So, in addition to its instability, people with Hb Hammersmith often have hemoglobin with a low oxygen affinity, causing cyanosis.
Oxyhemoglobin undergoes reversible oxygenation because its
heme iron is in the reduced (ferrous, Fe+2) state. However, this iron oxidizes spontaneously to the ferric (Fe+3) state and the resulting molecule, methemoglobin, cannot reversibly oxygenate.
The enzyme methemoglobin reductase returns the heme iron to the reduced (non-oxidized) state.
Several mutant globins ( or
or  ) with substitutions in the
heme pockets bond the heme in such a way as to resist the reductase.
These methemoglobins (Hb M) cause the heterozygote to be
cyanotic (but otherwise, asymptomatic) and have never been reported as a
homozygote (because it's probably embryonic lethal).
) with substitutions in the
heme pockets bond the heme in such a way as to resist the reductase.
These methemoglobins (Hb M) cause the heterozygote to be
cyanotic (but otherwise, asymptomatic) and have never been reported as a
homozygote (because it's probably embryonic lethal).
Methemoglobinemia can also result from a deficiency of the reductase enzyme but
this is autosomal recessive not autosomal dominant (and not a true pathology of the hemoglobins).
Subunit interactions are important in determining the hemoglobin's
affinity for oxygen. The  :
: interface shifts in a very specific way as the hemoglobin moves from the oxygenated (relaxed) to the deoxygenated (tense) state.
Substitutions along the
interface shifts in a very specific way as the hemoglobin moves from the oxygenated (relaxed) to the deoxygenated (tense) state.
Substitutions along the  :
: interface can prevent movement between the chains. Two examples illustrate this point.
interface can prevent movement between the chains. Two examples illustrate this point.
In Hb Kempsey ( 99 Asp->Asn) the mutation locks the hemoglobin
in the relaxed state producing a hemoglobin with a high oxygen affinity, so it won't easily release its oxygen to the tissues causing polycythemia.
99 Asp->Asn) the mutation locks the hemoglobin
in the relaxed state producing a hemoglobin with a high oxygen affinity, so it won't easily release its oxygen to the tissues causing polycythemia.
Conversely, Hb Kansas mutation ( 102 Asn->Thr) inhibits the formation
of the relaxed (oxygenated) structure, producing a hemoglobin that has a low oxygen affinity resulting in cyanosis.
102 Asn->Thr) inhibits the formation
of the relaxed (oxygenated) structure, producing a hemoglobin that has a low oxygen affinity resulting in cyanosis.
Collectively, thalassemias are the most common human single-gene
disorder. They are caused by a reduced amount of either the  or
or  protein, which alters the ratio of
protein, which alters the ratio of  to
to  , producing the pathology.
The excess (normal) chains precipitate, damaging the cell membrane and leading to premature destruction of the RBCs (like sickle cell disease - but different in its root cause).
, producing the pathology.
The excess (normal) chains precipitate, damaging the cell membrane and leading to premature destruction of the RBCs (like sickle cell disease - but different in its root cause).
When the amount of the  chain is reduced or absent, you have
chain is reduced or absent, you have  -thalassemia and when the
-thalassemia and when the  chain is not in high enough concentration you have
chain is not in high enough concentration you have  -thalassemia.
-thalassemia.
Mutant alleles for both thalassemias are found in high frequency and that's presumably caused by the protective advantage against malaria conferred on the carriers (like the sickle cell allele). Indeed, it is not impossible to have alleles for both types of thalassemias (as well as structural hemoglobin abnormalities) coexist in a person or population, leading to a wide variety of complex hematological problems and pedigrees.
Genetic disorders of  globin production affect the formation of hemoglobins in both fetuses and adults.
globin production affect the formation of hemoglobins in both fetuses and adults.
With no  globin chains to associate with, the
globin chains to associate with, the  globins form homotetrameric hemoglobin. The gamma tetramer (
globins form homotetrameric hemoglobin. The gamma tetramer ( 4) is known as Hb Bart's, and the beta tetramer (
4) is known as Hb Bart's, and the beta tetramer ( 4) is called Hb H. Neither of them is capable of releasing oxygen (under normal conditions) so severe intrauterine hypoxia (to the fetus) results and children are born with generalized fluid accumulation (hydrops fetalis).
4) is called Hb H. Neither of them is capable of releasing oxygen (under normal conditions) so severe intrauterine hypoxia (to the fetus) results and children are born with generalized fluid accumulation (hydrops fetalis).
The most common forms of  -thalassemias result from deletions,
probably promoted by the tandem arrangement of the two loci (
-thalassemias result from deletions,
probably promoted by the tandem arrangement of the two loci ( 1
and
1
and  2) which increases the frequency of unequal crossing over.
Deletions of one, two, three or all four genes cause a correspondingly more severe problem.
2) which increases the frequency of unequal crossing over.
Deletions of one, two, three or all four genes cause a correspondingly more severe problem.
The heterozygote state (two normal and two mutant genes) is called  -thalassemia trait and can occur two ways -
-thalassemia trait and can occur two ways - /-
/- or - -/
or - -/
 . The - -/
. The - -/
 is more common in Southeast Asians, and the
offspring of these "normal" carriers may receive two bad chromosomes (- -/- -) leading to hydrops fetalis. But parents of the (-
is more common in Southeast Asians, and the
offspring of these "normal" carriers may receive two bad chromosomes (- -/- -) leading to hydrops fetalis. But parents of the (- /-
/- ) genotype do not run that risk.
) genotype do not run that risk.
The  chain (unlike the
chain (unlike the  chain) is only important in the postnatal
period, so clinical onset occurs months (but less than two years) after birth and has similar pathology to
chain) is only important in the postnatal
period, so clinical onset occurs months (but less than two years) after birth and has similar pathology to  -thalassemias. But there are some features unique to
-thalassemias. But there are some features unique to  -thalassemia.
-thalassemia.
Hb A2 production continues (because the 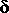 gene is intact). Indeed, elevated levels of Hb A2 are unique to
gene is intact). Indeed, elevated levels of Hb A2 are unique to  -thalassemia heterozygotes. The Hb F level is also increased because of selective survival (and perhaps increased production) of RBCs containing Hb F.
-thalassemia heterozygotes. The Hb F level is also increased because of selective survival (and perhaps increased production) of RBCs containing Hb F.
Most  -thalassemias (unlike
-thalassemias (unlike  -thalassemias) are due to single base-pair substitutions rather than deletions.
-thalassemias) are due to single base-pair substitutions rather than deletions.
There is great variety of  -thalassemia alleles and "homozygotes"
are more likely to be genetic compounds (compound heterozygotes).
-thalassemia alleles and "homozygotes"
are more likely to be genetic compounds (compound heterozygotes).
Individuals with a pair of bad thalassemia alleles ("homozygotes") have thalassemia major, a severe anemia requiring life-long
medical management.
When  globin is so low that no Hb A is present, the condition is
globin is so low that no Hb A is present, the condition is  0-thalassemia, but when some Hb A is detected the patient has
0-thalassemia, but when some Hb A is detected the patient has  +-thalassemia.
+-thalassemia.
Carriers of one  -thalassemia allele are clinically OK (but have
hypochromic, microcytic RBCs and slight anemia) and said to have
-thalassemia allele are clinically OK (but have
hypochromic, microcytic RBCs and slight anemia) and said to have  -thalassemia minor. Thalassemia minor can be diagnosed by hemoglobin electrophoresis, which reveals an increase
in the level of Hb A2 (
-thalassemia minor. Thalassemia minor can be diagnosed by hemoglobin electrophoresis, which reveals an increase
in the level of Hb A2 ( 2
2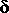 2).
2).
Regardless of the severity, survival into adult life was rare until recently.
Expansion of the bone marrow causes distinctive skeletal changes producing a typical face (the " -thalassemia face"). The RBCs are hypochromic and variable in size. Treatment is to correct the anemia and stabilize marrow expansion by blood transfusion, and control the iron accumulation with chelating agents. Bone marrow transplants may have some promise if done early.
-thalassemia face"). The RBCs are hypochromic and variable in size. Treatment is to correct the anemia and stabilize marrow expansion by blood transfusion, and control the iron accumulation with chelating agents. Bone marrow transplants may have some promise if done early.
Deletions are an infrequent cause of the disease but nearly every type of abnormality that can reduce the amount of the  globin has been identified as a cause of
globin has been identified as a cause of  -thalassemia. Nonfunctional mRNA mutations (frameshifts and premature stop codons) can cause
-thalassemia. Nonfunctional mRNA mutations (frameshifts and premature stop codons) can cause  0-thalassemia.
0-thalassemia.
However, the most common mutants are those that cause a decrease in the abundance of the normal  globin mRNA and they are caused by promoter mutations, RNA splicing mutants include splice junction mutants and cryptic splice sites and mRNA cap and tail mutants.
globin mRNA and they are caused by promoter mutations, RNA splicing mutants include splice junction mutants and cryptic splice sites and mRNA cap and tail mutants.
Large deletions that remove the entire  locus or other genes of the
locus or other genes of the  -globin
cluster produce thalassemias which are called "complex", and they are named according to the genes deleted (
-globin
cluster produce thalassemias which are called "complex", and they are named according to the genes deleted (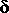
 0-thalassemia, A
0-thalassemia, A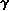
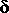
 0-thalassemia, etc.).
0-thalassemia, etc.).
Patients with deletions which leave at least one 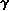 gene intact are viable because the remaining
gene intact are viable because the remaining 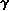 gene remains active! As a result Hb F (
gene remains active! As a result Hb F ( 2
2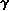 2) synthesis continues and these people have 100% Hb F in their postnatal hemoglobin.
2) synthesis continues and these people have 100% Hb F in their postnatal hemoglobin.
Patients with 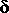
 0-thalassemia don't produce globin (no one knows why) and have a more severe condition (clinical thalassemia).
0-thalassemia don't produce globin (no one knows why) and have a more severe condition (clinical thalassemia).
Patients with A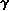
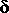
 0-thalassemia produce plenty of the other
0-thalassemia produce plenty of the other 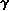 chain. They
are clinically benign and said to have hereditary persistence of fetal hemoglobin (HPFH).
chain. They
are clinically benign and said to have hereditary persistence of fetal hemoglobin (HPFH).
This work was created by Dr Jamie Love  and
and  licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
| Table of Contents | Homepage | How to get a FREE copy of the entire course (hypertextbook) | Frequently Asked Questions |