 and
and  licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
licensed under a Creative Commons Attribution-ShareAlike 4.0 International License. and
and  licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.

|
AIR |

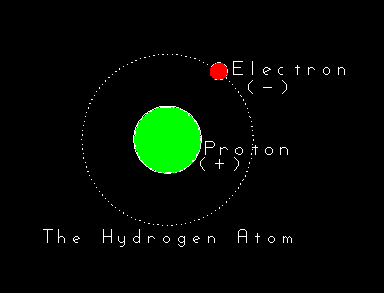
It's convenient to imagine atoms as tiny spheres around which orbit smaller spheres (dots!) of opposite electrical charge. This is a
sufficient and workable image on which to build an understanding of Modern Alchemy (chemistry). Be
warned, however, that this picture of atoms breaks down at the level of detail and understanding
required by physics.
|
|

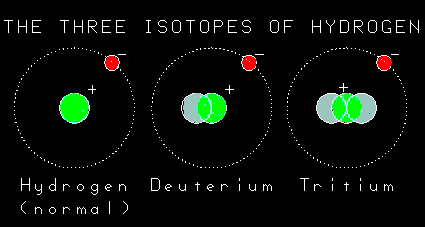
But they're all called hydrogen.
Ah, sort of. Because hydrogen is the most common element, people decided to give names to the hydrogen isotopes. It is fair to call all hydrogen isotopes hydrogen, but we can be more specific. If the hydrogen has one neutron, it is called deuterium and if it has two neutrons we call it tritium.And if it doesnít have any neutrons, just a proton, it is called hydrogen? |
|

| I think so. One of the neutrons in tritium turns into a proton and an electron. Because an extra proton is created, the nucleus now has two protons and so it is no longer the element hydrogen. It has been transmuted into the next element, helium. And an electron is spit out too, so there is a balance of charge. Because you canít get something from nothing! Even the mass is the same because the mass of the new proton plus the mass of the new electron equals the mass of the old neutron. Right? |

|



|
The Rock 370 bdps
|
times x |
The Conversion 1 Curie
|
equals
= |
The Answer (almost) 10 Curies
|
or
= |
The Answer (finally) 0.1 Specific activity
|

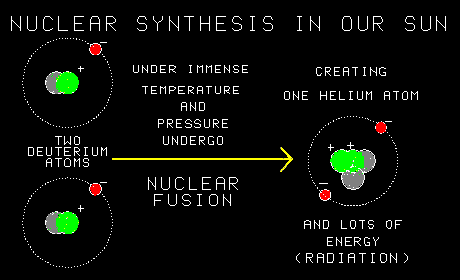
As the name implies, nuclear fusion is a reaction in which the nucleus of one atom fuses with the nucleus of another.I see. And by adding together two atoms, you add together all the protons that were in them. So you build heavier elements using the light ones.Yes. Exactly. In the process you also make an awful lot of energy. Lots of gamma rays!Sounds dangerous. And this still goes on? Inside stars? |
|

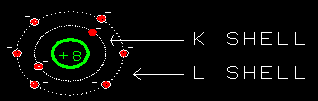
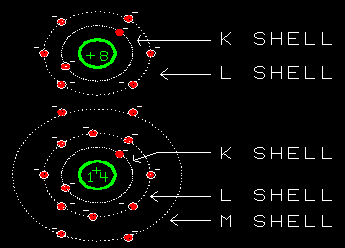
Yes, they do. Just outside the K-shell is the L-shell. It
can hold another 8 electrons. So oxygen's six remaining electrons
go into the L-shell.
|
|
I see. The electron shells of oxygen are arranged with 2 electrons
in the K-shell and 6 in the L-shell. With room for two more.
Yes, exactly. And that "room for two more" is very important, as you will see when we talk about bonding and molecules in the next Ancient Element, WATER. But let's stick with atoms.Bigger atoms have more shells to juggle
more electrons.
|
|

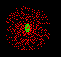
Right. BUT there is a certain probability that it is within a certain volume around the nucleus. We call this volume the electron cloud. Here's a drawing of a hydrogen atom's cloud. Each red dot represents the electron at one point in time. It is NOT to mean the atom has hundreds of electrons. It just has one electron that moves so much that it forms a cloud. |
|
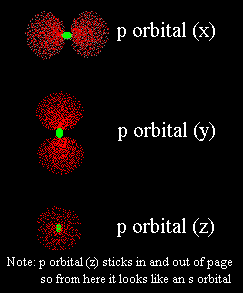
That's easy! S orbitals are spheres.
Right. Their electrons are likely to be found in any direction from the nucleus (that's a characteristic of an s orbital) so that makes a sphere. Those electrons are at a certain distance from
the nucleus (that's a characteristic of their shell).
Aren't they the only elements with K-shells as their outer (only)
shell?
|

|
What is it? "Orientations"? What's that all about?
What I mean is the figure 8 could be positioned in any one of the three dimensions.What are the three dimensions?Well, some people think of the three dimensions as "height, length and width". That's pretty good, but we Alchemists (and other scientists) call them "x, y and z". It has to do with a form of math called geometry.Ugh. Not math!Don't worry about it. Just imagine three different figure 8 lobes lined up in the three directions. One lies up and down (like the 8), another points sideways (left and right) and the third points in and out of your book. |
|
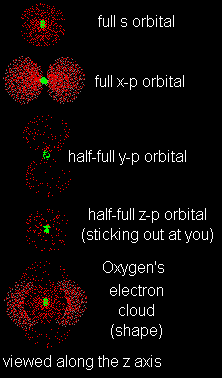
Just line them up and overlap them. Remember, the nucleus is at the center of ALL orbitals, so you can imagine laying one electron cloud from each orbital over the nucleus. When all the outer shell orbitals are overlapped, you get the complete shape of the atom!I think I see what you mean.I also see that the x-p orbital is full so it is twice as thick as the other two. Right, and that's an important point. Just because an orbital is only half-full, that DOESN'T mean the electron stays in just one lobe. It moves between the two lobes equally. Half-full orbitals are just less dense.I get it. When I overlap all of oxygen's L-shell orbitals I see a thick sphere in the center (the s orbital), a thick pair of lobes (the full x-p orbital) and another two pairs of lobes which are less thick. They are at right angles to the thick one.Yes, you've got it. Naturally it's hard to draw it well in only two dimensions, but I think you can imagine its shape. |
|


Course | Syllabus | Asked Questions | Homepage |
 and
and  licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.